Water pH is probably one of the critical aspects of water that most people never take into close account. It is an essential component of water that is usually ignored by most people. The pH of water is taken by the Geological Survey whenever they are conducting studies associated with water resources. Have you ever wondered the cause of a sudden change in the taste of water in your area? If so, this may be due to a change in the water pH.
What Exactly is Water pH and how can it be verified?
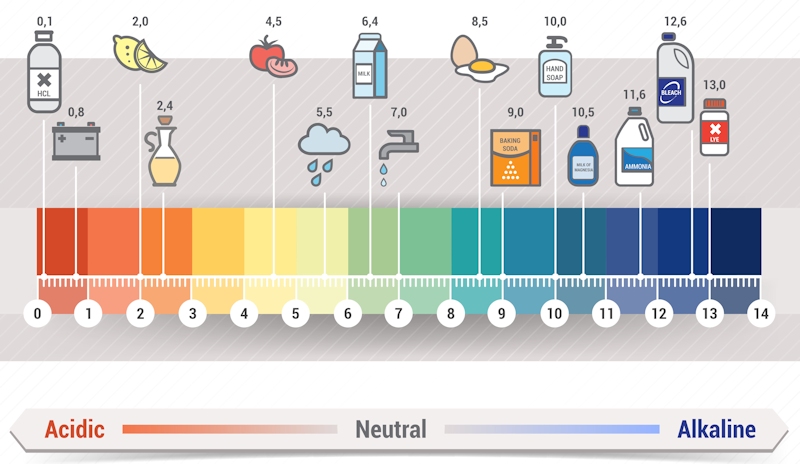
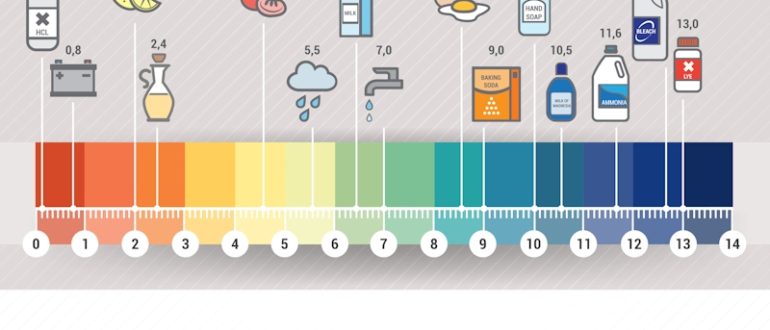
PH means the level of acidity or basicity of water. If you encounter more free hydroxyl ions in water, then the water is mostly basic. Conversely, water that has more free hydrogen ions is said to be acidic. The pH of water is a great indication of water that is rapidly changing in chemical composition. The main reason for this is because pH is profoundly affected by any chemicals that may be present in water. Every digit in a pH scale represents a 10-fold change.
What is the Importance of pH?
The importance of knowing the pH of water cannot be wished away. Did you know that it only by understanding the pH of water that you can determine its solubility as well as its biological availability? Solubility refers to the amounts that can be dissolved in the water whereas biological activity refers to the quantity of the water that can support aquatic life. Biological activity also refers to chemical constituents that can be supported by a water source. They may include carbon, nitrogen, phosphorous, copper and lead.
Procedure Used in Measuring Water pH.
The federal government has a responsibility to ensure the safety of citizens. This is guaranteed every year when the Geological Survey tests and analyzes thousands of water samples. The tests are either performed either at the lab or at the site. Various models are used for measurement. For instance, the smaller ones are typically taken out to the lab while the bigger ones are confined to the lab.
When pH meters are used for measuring pH, the glass probe located at the end of the retractable arm is placed in the water while the sample being tested is placed in a cup. Two electrodes that measure voltage are found in the glass bulb that is located at the end of the probe. One electrode is used to test the acidity of the same while the other one is immersed in a liquid with fixed acidity. The voltage is converted into pH upon which it is displayed on the screen.
In summary, pH is a factor that varies significantly across all regions not only in the United States but the world in general. Variation of pH exists in water bodies as well as in precipitations too. It is highly important that you be aware of the pH levels in your locality to avoid consuming contaminated water. After all, pH is basically the acidity or basicity of water and is measured using a pH scale.